
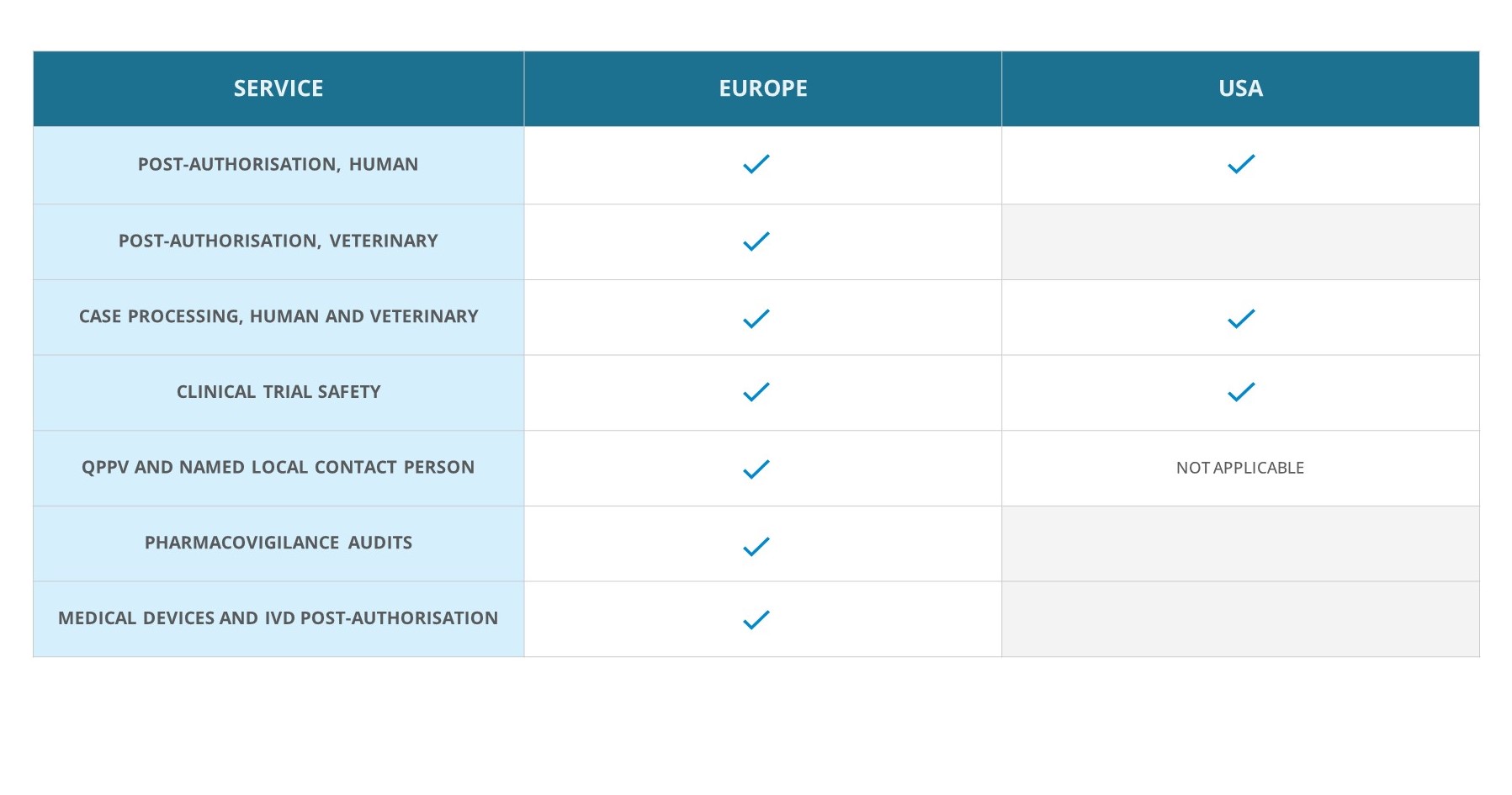
pharmacovigilance services for HUMAN and Veterinary Medicines AND vigilance serviceS FOR MEDICAL DEVICES
Medfiles is committed to ensuring the health and well-being of patients and consumers and to collaborating with clients to meet the increasingly complex regulatory requirements within the global pharmacovigilance landscape. We are specialised in pharmacovigilance and vigilance services concerning human and veterinary medicinal products and medical devices. Whether you need help at headquarters or local level, our experts are happy to help.
We will guide you through the entire product lifecycle, from safety profiling in the clinical phase to post-market PV systems. Our pharmacovigilance services are integrated with our medical information, regulatory affairs and clinical trial services, which means that you can benefit from our efficient multi-disciplinary teamwork in all tasks outsourced to us. Our services are always tailored to your needs, from comprehensive service packages to individual ad hoc tasks.
Pharmacovigilance throughout the product life cycle
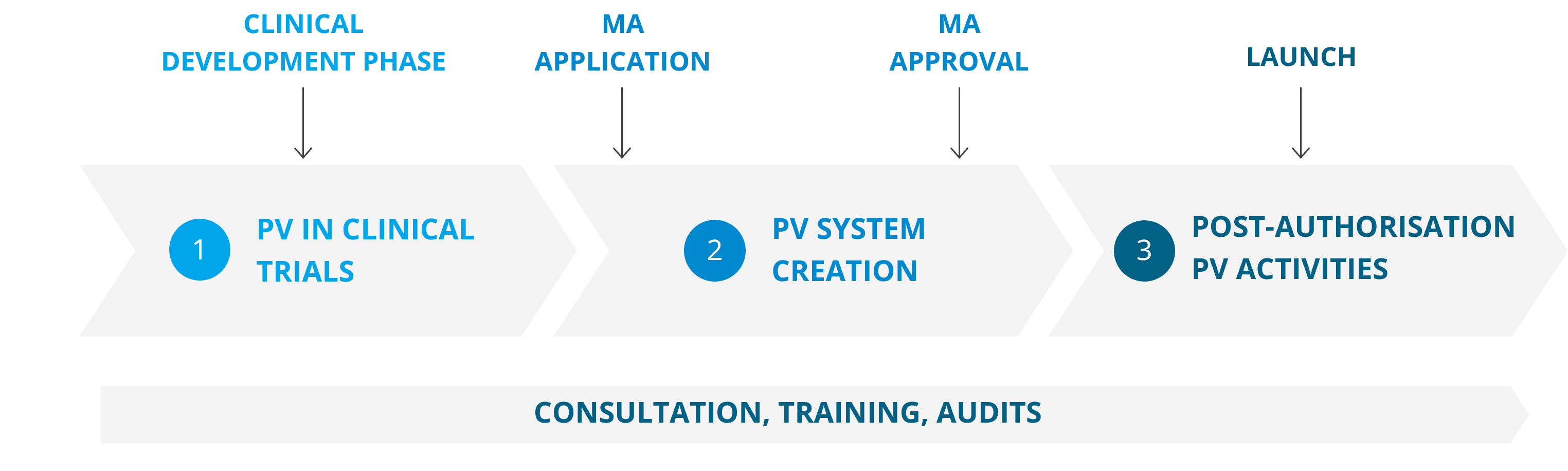
The Medfiles Pharmacovigilance Team of safety experts and safety physicians consists of skilled professionals in Europe and the US. By choosing to work with us, you can be sure that your pharmacovigilance system is always compliant and all the applicable requirements are met. To ensure the high quality of our services, our experts undergo continuous training and are always up to date with the latest requirements and practices.
Pharmacovigilance and vigilance services
EU QPPV, local QPPV and named safety contact
QPPV team with extensive experience in PV systems.
Human post-authorisation pharmacovigilance
Comprehensive up-to-date PV systems for human medicines.
ICSR
case processing
Triage, data entry, medical evaluation and expedited reporting.
Vet post-authorisation pharmacovigilance
Comprehensive up-to-date PV systems for veterinary medicines.
Clinical trial safety
Monitoring product safety during the clinical development phase ensures safe products on the market.
Medical device and IVD vigilance
Extensive experience and know-how of the Medfiles Vigilance and MD Team at your service.
Medical information
Efficient team with in-depth understanding of adverse events (AEs) and product quality complaints (PQC).
Geographical presence of pharmacovigilance and vigilance services
Over 20 years of experience in pharmacovigilance
65+
clients in 15 countries (eu, usa, asia)
6000+
Medicinal products monitored
4000+
Medical enquiries
150+
Aggregate reports

Free WEBINAR: EU Veterinary Medicinal Products Regulation from the perspective of variations and the quality part of the pharmacovigilance system
In our webinar you will learn about the main changes and requirements brought about by the Veterinary Medicinal Products Regulation. In the webinar we will discuss variation management and the relationship between pharmacovigilance systems and the related quality measures.
Pharmacovigilance team leaders

Satu Kujala
Head of Pharmacovigilance
Satu Kujala has worked at Medfiles since 1999 acting in various leadership positions. She is currently serving as Head of the Pharmacovigilance team consisting of 20 experts in Finland and Baltics.
Satu has a Licentiate’s degree in Philosophy (pharmacy), and she has specialised in pharmacology and industrial pharmacy. She graduated from the University of Helsinki. After a short career in the university and pharmacy, she has worked within the pharmaceutical industry for 30 years and has gained diverse experience through working on marketing, quality, wholesale, clinical trials, safety and regulatory tasks. She is familiar with Good Clinical, Pharmacovigilance and Distribution Practices (GCP, GVP and GDP). Acting as EU QPPV for sixteen years has given her deep knowledge and experience of pharmacovigilance systems.

Saara Mikkola
Head of Operations, Pharmacovigilance, EU QPPV
Saara Mikkola joined Medfiles Pharmacovigilance team in 2020 as a Drug Safety Expert, and in 2022 she was promoted to Safety Manager and later to Head of Operations, Pharmacovigilance. She works as an EU QPPV for Medfiles and has her focus on post-market pharmacovigilance services.
Saara has a master’s degree in pharmacy and graduated from the University of Helsinki, Finland, in 2012. She has worked within the pharmaceutical industry for over 10 years with her focus on pharmacovigilance. Saara has gained her expertise through diverse pharmacovigilance tasks and responsibilities. She is well-versed in the operational aspects of pharmacovigilance, including case processing, quality management, compliance with regulatory requirements (EU regulation), and maintaining pharmacovigilance systems.
She is adept at working in cross-functional teams, collaborating with different stakeholders, and adapting to the ever-evolving pharmacovigilance landscape and technologies.
Contact us
You may also be interested in:
- Free webinar: EU Veterinary Medicinal Products Regulation from the perspective of variations and the quality part of the pharmacovigilance
- Global pharma company & Medfiles: Cooperation in obtaining and maintaining marketing authorisations in the EU
- Bimeda & Medfiles: a decade of partnership in veterinary regulatory affairs in the EU
- Blog: Are we really performing signal management – or just think we are doing it?
- A team of 12 experts ensures high-quality regulatory services in the Baltic countries
